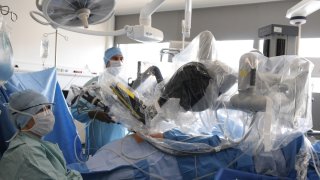
A robotic device burned and tore a woman’s small intestine while she was undergoing surgery for colon cancer, according to a wrongful death lawsuit filed this week in Florida.
The woman, Sandra Sultzer, developed abdominal pain and fever after her surgery in September 2021 and needed additional procedures to close the tear, the lawsuit said. The suit said she died in February 2022 because of the small intestine injury.
Her husband, Harvey Sultzer, is suing the maker of the device, Intuitive Surgical, for damages.
The suit alleges that Intuitive Surgical knew the robot had insulation problems that might cause electricity to leak out and burn internal organs but didn’t disclose that risk to the Sultzers or the public.
Get DFW local news, weather forecasts and entertainment stories to your inbox. Sign up for NBC DFW newsletters.
It also claims that Intuitive sells its robots to hospitals that have no experience in robotic surgery and doesn’t properly train surgeons in how to use the device, known as the da Vinci. Intuitive does offer a training program, but an NBC News investigation in 2018 found that it can’t legally require surgeons to complete it.
Intuitive Surgical didn’t respond to a request for comment. Jack Scarola, a Florida attorney representing Harvey Sultzer, said neither he nor his client had any comment beyond the allegations in the suit.
Issues of such a nature go back more than a decade, according to the lawsuit.
U.S. & World
The suit alleges that Intuitive has received thousands of reports about injuries and defects associated with the da Vinci but has “systematically underreported” injuries to the Food and Drug Administration.
A 2014 financial report that Intuitive filed with the Securities and Exchange Commission said the company at the time was a defendant in around 93 lawsuits.
The plaintiffs in about 93 suits "allege that they or a family member underwent surgical procedures that utilized the da Vinci Surgical System and sustained a variety of personal injuries and, in some cases, death as a result of such surgery," the company wrote.
The company added that it also set aside $67.4 million in the first quarter of 2014 and $9.6 million in the second quarter so it could settle a number of product liability claims.
A case that went to trial in 2017 involved the use of the da Vinci robot during a woman’s hysterectomy. Intuitive said the claim was without merit, and it was settled for an undisclosed amount. A jury previously ruled in favor of Intuitive in a case in 2013 that alleged the company failed to provide sufficient training to doctors.
In its 2023 annual report, the company told the SEC that it was a defendant in "a number of individual product liability lawsuits" involving the same allegations.
Many doctors consider robotic surgery to be safe, but there’s debate about whether it’s more effective than traditional surgery. The technology is meant to make procedures more precise and less invasive, which in theory would lead to shorter and less painful recoveries.
Intuitive introduced the first version of the da Vinci system in 1999, when robotic surgery was still relatively novel. The FDA approved the system a year later.

According to Sultzer’s lawsuit, Intuitive received hundreds of complaints and reports about its da Vinci robot from July 2009 to December 2011. Many of the reports mentioned cracks or slits on a rubber tip used to cover da Vinci’s metal instruments, the suit said, adding that the defects allowed electricity to escape without surgeons’ knowledge.
In 2011, researchers from the University of Western Ontario flagged safety concerns with the device’s surgical instruments in a study. The researchers tested 37 instruments and found that all of them had “energy leakage,” which in some cases they determined was sufficient to cause electrical burns.
Then, in July 2013, the FDA sent a warning letter to Intuitive, citing several examples of its failure to comply with federal regulations. One of the examples involved the company’s sending letters to clients about how to use its tip covers — a response to complaints and reports of patient injuries.
This story first appeared on NBCNews.com. More from NBC News: