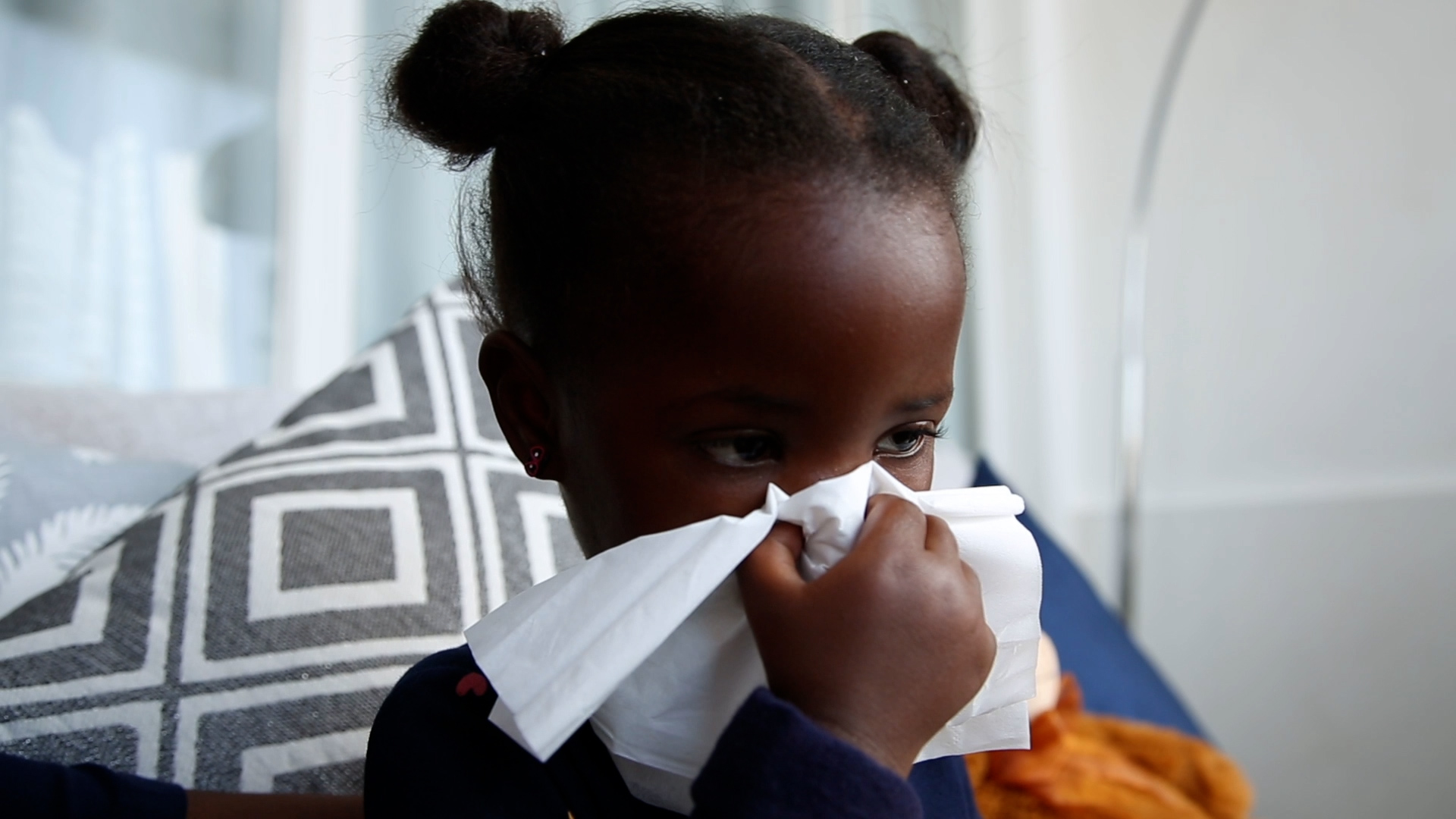
The Centers for Disease Control and Prevention alerted doctors nationwide Monday about a limited availability of certain doses of a newly approved antibody drug given to infants to prevent RSV infection.
Cases of RSV, or respiratory syncytial virus, have started to rise as cold and flu season begins.
The Food and Drug Administration approved the antibody drug, called Beyfortus, in July. It is not a vaccine, but it acts similarly to one: Instead of prompting the immune system to develop its own antibodies to RSV, it delivers antibodies directly to the blood via injection.
Get DFW local news, weather forecasts and entertainment stories to your inbox. Sign up for NBC DFW newsletters.
Newborns and infants can get doses of Beyfortus during their first RSV seasons, and children up to age 2 who are at high risk for severe illness from the virus can get second doses during their second RSV seasons.
According to the CDC alert, the highest dosage, 100 milligrams, is in limited supply. The agency told doctors to prioritize getting those doses to infants at the highest risk of severe RSV, including infants younger than 6 months and those with underlying conditions. The CDC also advised doctors to preserve 50 mg doses for infants who weigh less than 11 pounds.
Read the full story on NBCNews.com.